
Learn to conduct a meaningful hazard analysisĮdwin Waldbusser retired from industry after 30 years in management of development of medical device products and development of company Quality Systems. we will explain these concepts and provide examples so that the process is clear.Įxplanation of Hazard Analysis terms hazard analysis process explanation using a template examples of terms will be given hazard analysis examples will be covered step by step
#Risk analysis iso 14971 template iso
Hazard Analysis is the most powerful of the risk management tools described in ISO 14971 but it is very confusing. FDA recommends using ISO 14971 as a guide and has accepted it as a recognized standard.
#Risk analysis iso 14971 template how to
We will explain how to integrate Human Factors studies into the Hazard Analysis and how to integrate Hazard Analysis into the design program.įDA expects that as part of a product development program risk management will be conducted. We will go step by step through a typical hazard analysis. Also, how to deal with residual risk will be discussed. Examples of hazards and hazardous situations will be discussed. We will go step by step through a template for hazard analysis so that the process is clear. The confusing terms “hazard”, hazardous situation”, “harm”, “causative event”, “ALARP”, “risk index”, “residual risk” will be explained. In this ISO 14971 risk management training we will explain in detail the process of conducting a hazard analysis.
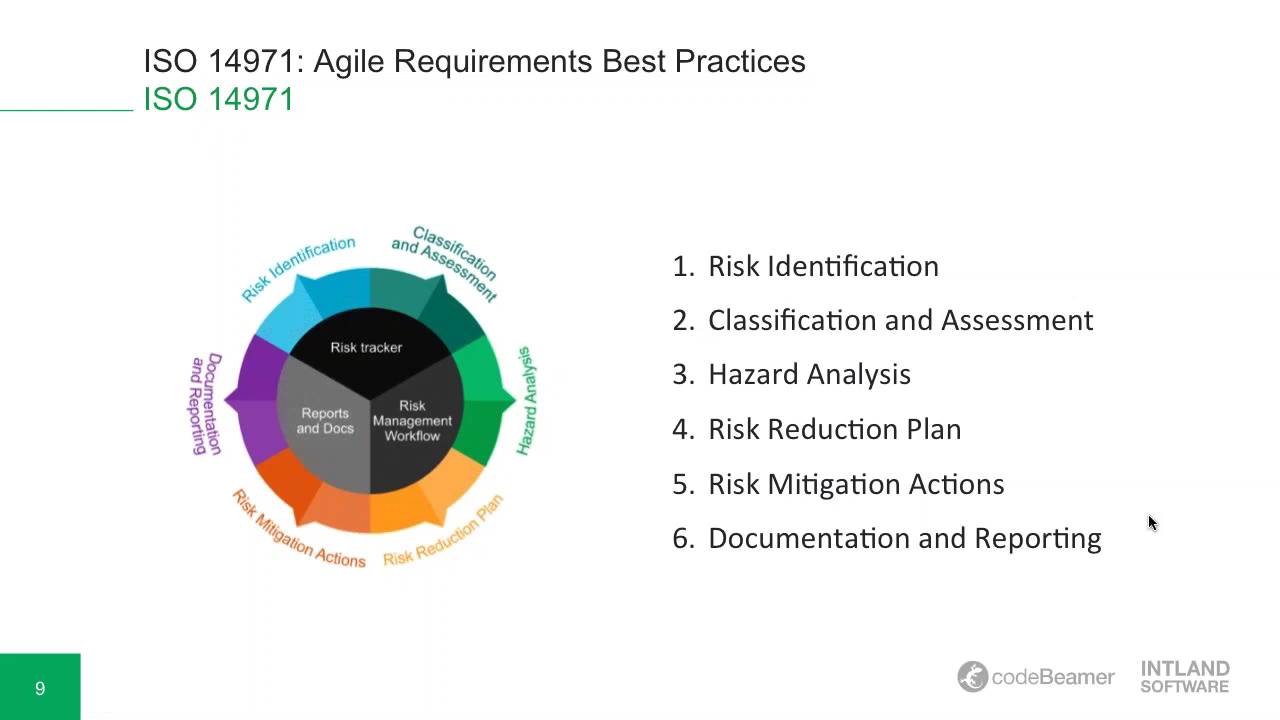
FMEA and FTA consider only fault conditions and are more suited as reliability tools than as product safety tools. This is the most powerful of the risk management techniques because it considers risks in normal operation as well as fault conditions. One of the techniques described in ISO 14971 is Hazard Analysis. Table 8 - Risk control measures for Palpreast useĪfter the Risk control all risks must be considered acceptable.The US FDA expects that as part of a product development Design Control Program risk management will be conducted.įDA recommends using ISO 14971 as a guide and has accepted it as a recognized standard. The risk control measures related to Palpreast use are described in Table 8. The green cells are related to acceptable risk, the red cells are relative to unacceptable riskĪccording to ISO 14971, the “Risk control” is defined as the “Process in which decisions are made and measures implemented by which risks are reduced to, or maintained at, specified levels”. Table 7 - Semi-qualitative 5x5 risk evaluation matrix. Table 7 shows the risk evaluation and risk acceptability for Palpreast. For each identified hazardous situation, the manufacturer decides if risk reduction is required, on the basis of its acceptability criteria defined in the risk management plan. Table 6 - Semi-quantitative 5x5 risk matrix for Palpreast Evaluation RiskĪccording to ISO 14971, the “Evaluation of Risk” is defined as the “Process of comparing estimated risk against given risk criteria to determine acceptability of the risk”. Table 4 - Semi-qualitative probability levels Table 3 - Five qualitative severity levels

harm : physical injury or damage to health of person, or damage to property or environments.hazardous situation : circumstance in which people property, or the environment are exposed to one or more hazard(s).hazard : potential source of harm to patient or user.Risk analysis is defined, according to ISO 14971, as the “Systematic use of available information to identify hazards and to estimate the risk”, where This section was developed step-by-step, to be used also a teaching material and as template for other projects developed through UBORA.Ī complete risk analysis has been performed taking into consideration Palpreast device.


 0 kommentar(er)
0 kommentar(er)
